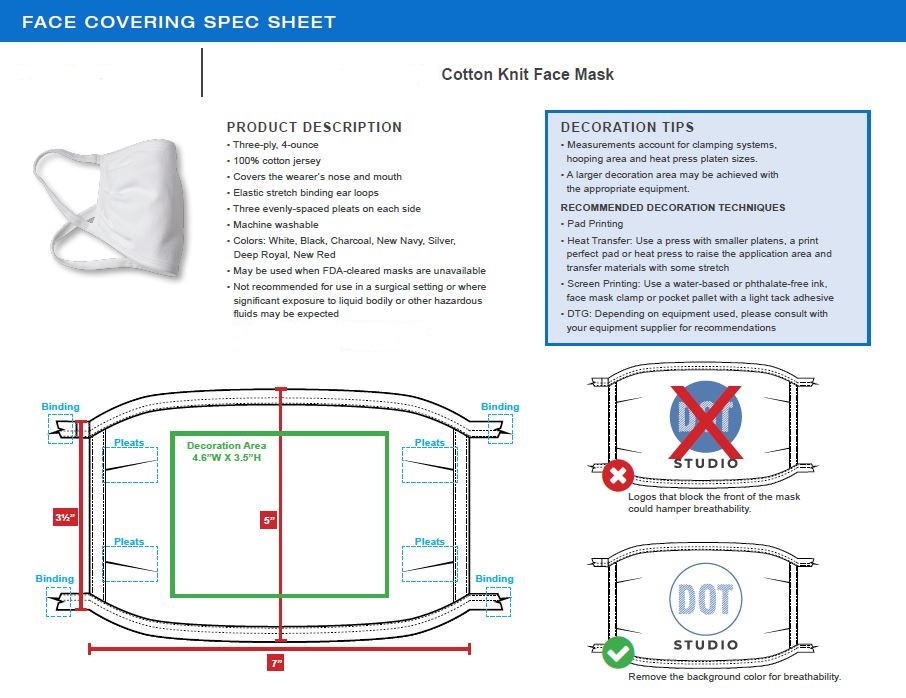
Designed for maximum comfort, this Cotton Knit Face Mask goes beyond standard cotton masks thanks to our partnership with Sciessent, a U.S.-based global provider of antimicrobial solutions, to incorporate its Agion® treatment into the fabric. Learn more about Sciessent. This three-ply, 100% cotton jersey face mask covers the wearer’s nose and mouth. Elastic stretch binding ear loops help hold it comfortably in place. •Three-ply 4-ounce, 100% cotton jersey
Cotton Knit Face Mask 3-Ply pack of 5 Masks
$15.00
Description
Designed for maximum comfort, this Cotton Knit Face Mask goes beyond standard cotton masks thanks to our partnership with Sciessent, a U.S.-based global provider of antimicrobial solutions, to incorporate its Agion® treatment into the fabric. Learn more about Sciessent. This three-ply, 100% cotton jersey face mask covers the wearer’s nose and mouth. Elastic stretch binding ear loops help hold it comfortably in place. •Three-ply 4-ounce, 100% cotton jersey
•Fabric touching skin: 100% cotton
•Mask contains silver and copper
•Three evenly-spaced pleats on each side
•May be used when FDA-cleared masks are unavailable
•Machine washable
•Non-returnable
•Not recommended for use in a surgical setting or where significant exposure to liquid, bodily or other hazardous fluids may be expected and where the infection risk level through inhalation exposure is high.
•The product has not been FDA cleared or approved.
•The product has been authorized by FDA under an EUA for use by HCP as PPE to help prevent the spread of infection or illness in healthcare settings and by the general public to help slow the spread of the virus during the COVID-19 pandemic.
•The product has not been FDA cleared or approved.
•The product has been authorized by FDA under an EUA for use by HCP as PPE to help prevent the spread of infection or illness in healthcare settings and by the general public to help slow the spread of the virus during the COVID-19 pandemic.
•This product is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices, including alternative products used as medical devices, during the COVID-19 outbreak, under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1) unless the authorization is terminated or revoked sooner.
Additional information
| Weight | .25 lbs |
|---|